Paediatric Acute-onset Neuropsychiatric Syndrome (PANS) and intravenous immunoglobulin (IVIG): comprehensive open-label trial in ten children
Hajjari, P., Oldmark, M. H., Fernell, E., Jakobsson, K., Vinsa, I., Thorsson, M., Monemi, M., Stenlund, L., Fasth, A., Furuhjelm, C., Johnels, J. Å., Gillberg, C., & Johnson, M.
A new study published in BMC Psychiatry discusses an in depth open-label trial on PANS patients who received IVIG treatment 2 g/kg monthly for three months.
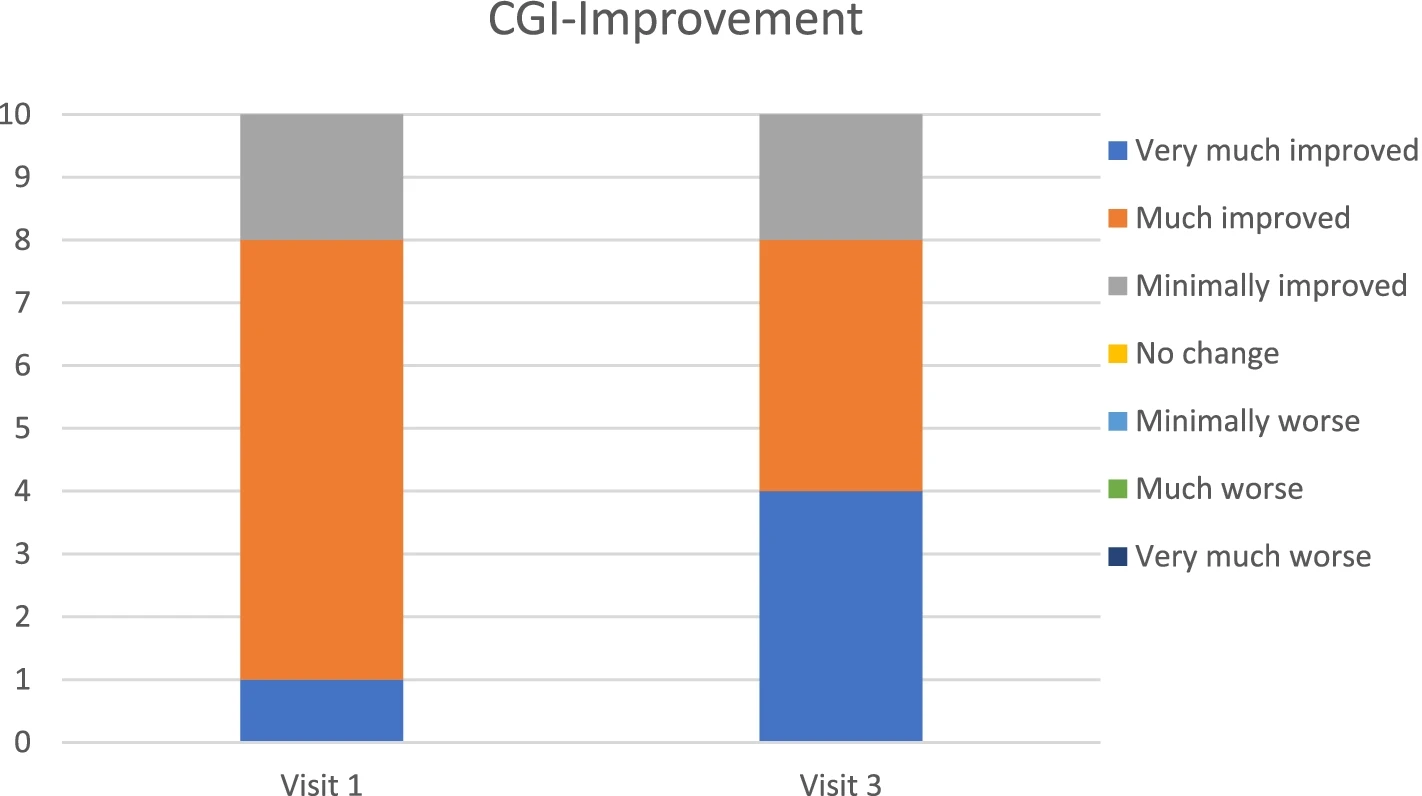
This open-label prospective IVIG treatment trial in 10 children with PANS demonstrated substantial improvements in PANS symptom severity and impairment (including OCD symptoms), global functioning and school attendance after 3 monthly IVIG treatments. From severe illness at baseline, 9 patients were clinical responders with > 30% improvement, and 7 patients improved to mild illness or remission.
According to the authors, this is the first IVIG study to report broad baseline and follow-up data on global severity and functioning and detailed symptom development according to the defined symptom criteria of PANS.
Download the open access paper on PANS/PANDAS for free at https://bit.ly/3vNHkcs.